
Orphan Drug Designation for Marketing
04 january 2024

What is Orphan drug designation
The intention must be for the treatment, prevention, or diagnosis of a disease that is rare disease or life-threatening, or chronically debilitating;
Benefits of Orphan drug designation:
Orphan drug designation is a status granted by regulatory agencies to pharmaceuticals and biologics developed to treat rare diseases or conditions. Here are some benefits of orphan drug designation:
1. Market Exclusivity
2. Financial Incentives
3. Extended Patent Protection
4. Accelerated Approval
5. Grants and Funding
6. Public Recognition
7. Assistance with Clinical Trials
8. Higher Pricing Flexibility
9. Potential for Future Expansion
The criteria for orphan drug designation:
- The prevalence of the condition in the EU (not more than 5 in 10,000) and in the United States (fewer than 200,000 people)
- The drug should focus on a significant unmet medical need in patients suffering from rare diseases. Therefore, existing treatments are either unavailable or insufficient, or they should show that they may offer a major therapeutic advantage over existing drugs or that there are no alternative treatments for them.
Note: These criteria are based on the guidelines provided by the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other similar agencies.
Marketing authorization stage
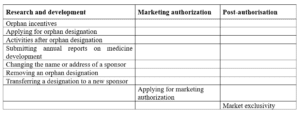
- The MA application is submitted to EMA for assessment through the CP procedure and assessed by EMA’s Committee for Orphan Medicinal Products (COMP).
- These products are eligible for conditional marketing authorization.
- Sponsors should apply for maintenance of the orphan designation to be eligible for the ten-year market exclusivity incentive.
- Sponsors also submit an evaluation of orphan similarity.
- Benefits such as further incentives, including administrative and procedural assistance from the Agency’s SME office and fee reductions, are encouraged by the agency developing orphan medicines.
After orphan designation
- Sponsors who obtain orphan designation benefit from protocol assistance, a type of scientific advice specific to designated orphan medicines, and market exclusivity once the medicine is on the market. Fee reductions are also available depending on the sponsor’s status and the type of service required.
- Sponsors must submit an annual report to the agency summarising the status of the development of the medicine.
- Image facts:
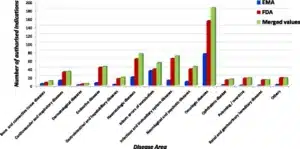
Distribution of orphan designations per disease area
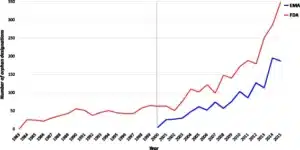
Orphan designations in the EU and US released per year
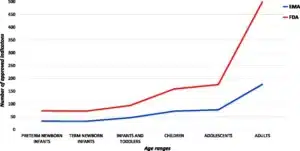
Distribution of indications in the EU and US per age groups. Legend: label not available for 7 ODDs
How PLG can support your orphan drug applications
We at PLG have an efficient team of experts who cannot only guide you through the possibilities and advise you for the best outcome but can file submissions for orphan drug designation and help at every stage required.
We at PLG have an efficient team of experts who cannot only guide you through the process and the possibilities and advise you for the best outcome but also file submissions for orphan drug designation and help at every stage required. Our clinical and regulatory experts will support you in designing a scientifically comprehensive and regulatory-compliant dossier for your planned orphan designation. Our experts can also support you in managing any regulatory meetings related to the ODD. We can also hold and maintain the Orphan drug designation on your behalf if required.
References:
Register to our news and events
Go to our Events to register
Go to our News to get insights
