The five key steps of the Quality by Design systematic approach for drug development
13 october 2022

Introduction - Elements of pharmaceutical quality by design
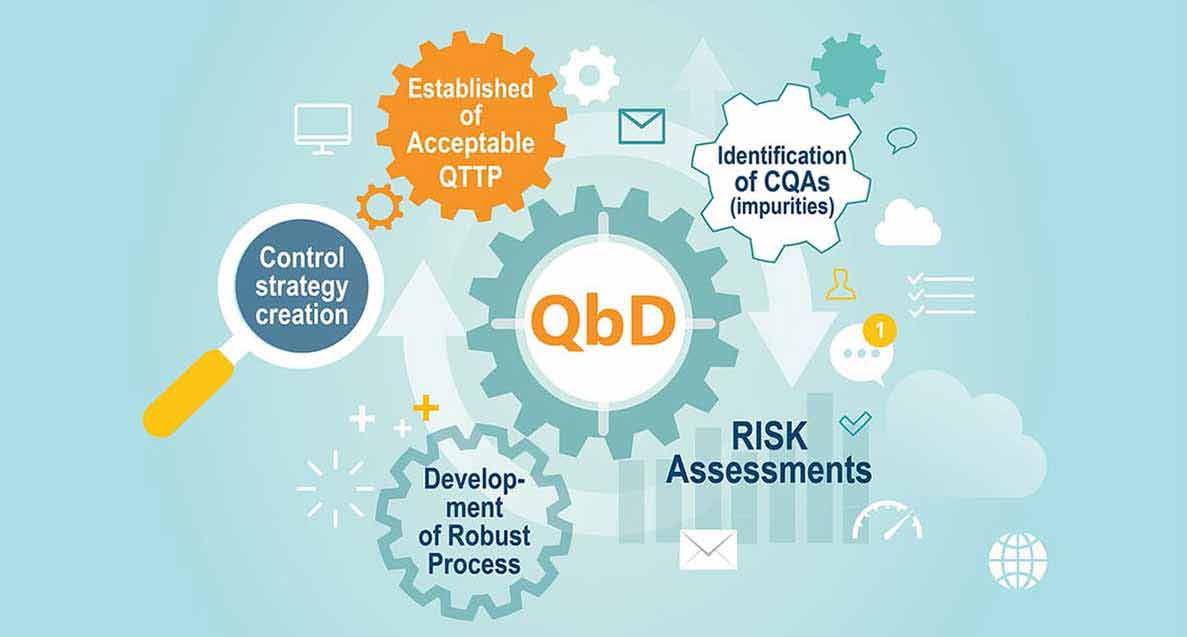
The five key steps of the QbD systematic approach for drug development are as follows:
- Definition of the quality target product profile (QTPP)
- Identification of the drug product critical quality attributes (CQAs)
- Risk assessment to identify which material attributes and/or process parameters affect the CQAs
- Determination of the functional relationships between material attributes and/or process parameters and CQAs (e.g., Design Space)
- Use of the enhanced product/process understanding, in combination with quality risk management, to establish a control strategy.
STEP 1 - Definition of the QTPP
The QTPP forms the basis of design for the development and should consider the intended clinical use, dosage strength, container closure system, as well as all the drug product quality criteria/attributes appropriate for the intended type of medicine (e.g. dissolution, sterility, purity, stability and drug release).
STEP 2 – Identification of the drug product CQAs
A CQA is a physical, chemical, biological, or microbiological property that is considered critical to obtain the desired quality profile, and therefore should remain within an appropriate limit, range, or distribution (e.g., disintegration time and dissolution are CQAs for tablets, such as aerodynamic properties and sterility for parenteral and inhaled products, respectively).
CQAs play a pivotal role for product and process development, becoming the reference to identify at an early stage any potential quality issue in the manufacturing and/or control process. In line with ICH Q9 and Q10, CQAs are derived from QTPP and identified by the application of quality risk management through an iterative process, assessing the extent to which their variation can impact the drug product quality profile.
STEP 3 – Product/process design and understanding: identify how material attributes and process parameters impact CQAs using Risk Assessment
Risk assessment is guided by ICH Q9 and typically performed early in the pharmaceutical development to identify which material attributes and/or process parameters could affect CQAs (e.g., for tablets, the dissolution CQA could be affected by the particle size distribution of the drug substance and/or excipients). The list of potential parameters could be quite extensive, so risk assessment methods are adopted to explore and rank them based on prior knowledge and experimental data. Once identified, they can be further studied to achieve a higher level of process understanding, setting the stage for the establishment of the design space.

STEP 4 - Design space: enhanced regulatory flexibility
The risk assessment and process development experiments (design of experiments) lead to a strong understanding of the linkage and effect of some process parameters/material attributes on product CQAs, enough to identify ranges within which consistent quality can be achieved (i.e., design space ranges). These parameters/attributes can then be selected for inclusion in the design space, and their operating range can vary within the enhanced design space ranges approved by competent authorities without any regulatory action, improving manufacturing flexibility.

Need help? We can support you!
As a European-based company with experience in delivering advice and regulatory compliance services for development of pharmaceutical products, ProductLife Group can support you at all stages of the Quality by Design development process, offering strategic advice, resources, and expertise to support drug development as well as regulatory/CMC submissions.
Contact us to find out more here.
Register to our news and events
Go to our Events to register
Go to our News to get insights
