
Harnessing the similarities of US and European Clinical Trial Applications
30 november 2022

Clinical trial applications in the US and Europe follow market-specific processes, but the overlap in information requirements is not as straightforward as it might seem.
Investigational New Drug (IND) application is the document submitted to the FDA to obtain regulatory approval to run clinical trials in the United States. Where an EU Clinical Trial Application (CTA) already exists then the Investigational medicinal product dossier (IMPD) is often one of the key documents, along with other study reports and investigator’s brochure (IB), used as a source of information to start preparing an IND. An IND follows the Common Technical Document (CTD) structure developed by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH); requiring detailed product and development data, including information on manufacture, data from non-clinical studies to clinical studies, as well as previous relevant clinical studies. It’s also necessary within an IND to provide comprehensive source documentation, including study reports.
From May 2018, it became mandatory to submit an IND in the eCTD format, however, an IMPD can be submitted using specific templates provided by EMA. Further details of the requirements are available from our blogs on the topic, implementation of the EU Clinical Trial Regulation.
For example, quality template for IND in eCTD is 3.2.S for drug substance and 3.2.P for drug product part whereas quality template for IMPD is 2.1.S for drug substance and 2.1.P for Medicinal Product. Both the templates for IND and IMPD are presented below as Figure 1 and Figure 2, respectively.
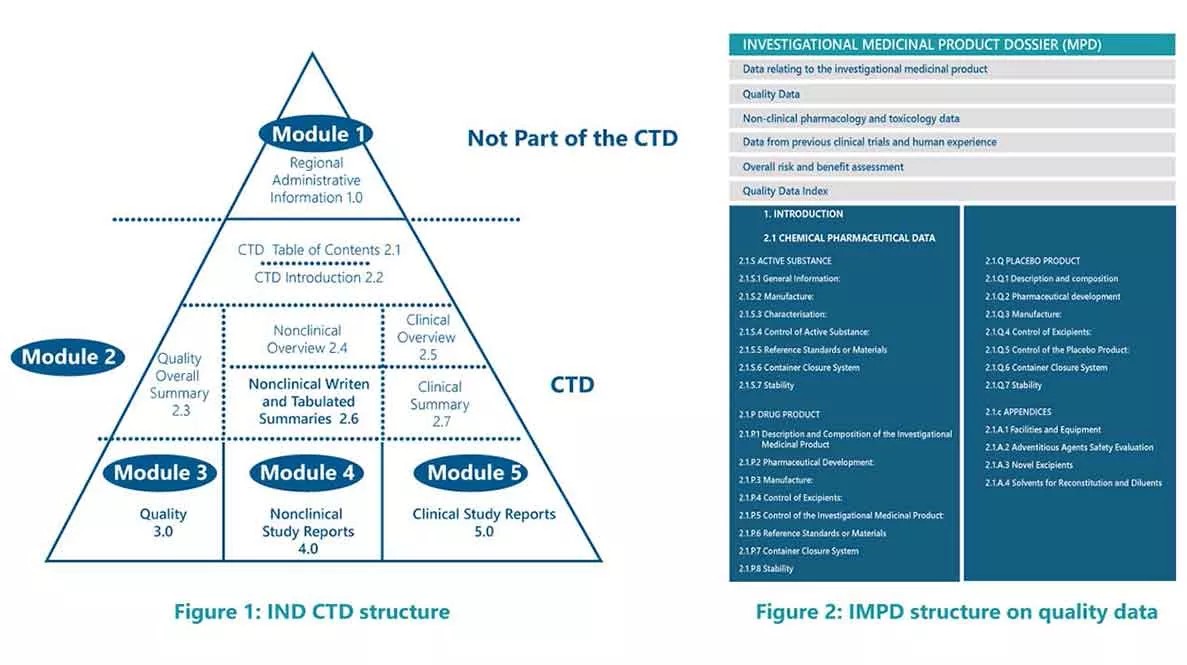
Investigational Medicinal Product Dossier (IMPD) is the main document of a CTA submitted to regulatory authorities within the European Union (EU) to obtain authorization for conducting a clinical trial with an Investigational Medicinal Product (IMP) in the Member State(s).
For an IMPD preparation, a concise high-level summary of details concerning the quality, chemistry, manufacturing, and controls (CMC) of a drug is a critically important aspect in securing clinical trial approval by the European regulatory authorities.
Some pharmaceutical companies usually use the Investigational New Drug (IND) application as a reference when writing the EU IMPD, despite the structure being different. This can lead to the writer overlooking, and ultimately failing to meet the requirements set by the European guidelines, which can result in serious objections by EU regulators and the potential rejection of the clinical trial application.
The IMPD is crucial not only for clinical trial submissions, but also to support future marketing authorization applications. A precise and complete IMPD is essential for all the stages of a drug development program.
When comparing the EU with US trial application processes, the differences between the IMPD and IND structures include:
- In case of IMPD it requires only high level summary for introductory sections of the clinical development program of an IMP, whereas the IND requires extensive details.
- As the product advances in clinical development, new data can be added to the IND whereas a new or updated IMPD application must be submitted for each CTA in the EU.
- The IMPD consists of the following 2 parts:
- The Quality section which includes information on the active medicinal product, placebo, and reference medicines (if applicable).
- The Safety and Efficacy section which includes a summary of data from all clinical and non-clinical studies, with an overall assessment of the risks and benefits. In this section, reference can also be made to the Investigator’s Brochure (IB).
However, an IND application must include information in three broad areas:
-
- Animal Pharmacology and Toxicology Studies – Includes pre-clinical data to demonstrate that the product is safe for initial testing in humans.
- Manufacturing – Information pertaining to the composition, manufacturer, stability, and controls used for manufacturing the drug substance and the drug product. This information is assessed to ensure that the company can adequately produce and supply consistent batches of the drug.
- Clinical Protocols and Investigator Information – Detailed protocols for proposed clinical studies to assess whether the initial-phase trials will expose subjects to unnecessary risks. Also, information on the qualifications of clinical investigators–professionals (generally physicians) who oversee the administration of the experimental compound to assess whether they are qualified to fulfill their clinical trial duties. Finally, commitments to obtain informed consent from the research subjects, to obtain review of the study by an institutional review board (IRB), and to adhere to the investigational new drug regulations.
Preparing an IND based on an IMPD – and vice versa – is not a copy and paste exercise. There are unique questions that typically arise when writing any IMPD or IND. General guidelines for writing an IMPD and IND can be referred to from the EMA and USFDA websites, however tapping into expertise can help avoid common mistakes. There is often room to improve the description of the manufacturing processes and related documentation in advance, resulting in shorter time to regulatory approval.
Keen to be efficient and get your clinical trial applications right first time? We can support you!
With decades of experience in delivering strategic advice on EU and US regulatory requirements, ProductLife Group provides practical support for CMC and effective writing of INDs and IMPDs. Alongside our strategic advice, we offer tactical resources, and expertise to address questions raised by health authorities and regulatory bodies.
Register to our news and events
Go to our Events to register
Go to our News to get insights
