The role of Artificial Intelligence in CMC and Regulatory Affairs for 2025
06 october 2025

Abstract
AI is significantly impacting the pharmaceutical industry, particularly in CMC and RA.
This article explores the foundational concepts of AI, categorized by capability (Narrow AI, Artificial General Intelligence, Artificial Super intelligence) and functionality (Reactive Machine AI, Limited Memory AI, Theory of Mind AI, Self-Aware AI), alongside Generative AI’s capabilities in creating novel content such as text, images, and data. Key benefits of Generative AI include enhanced customer experience, improved decision-making, operational efficiency, cyber security, employee productivity, competitive analysis, and demand prediction. However, it poses risks like data biases, IP infringement, hallucinations (producing false yet plausible information), and ethical concerns around fairness, transparency, accountability, and social benefit.
In CMC, AI automates Module 3 preparation, the drafting of response documents, ICH guideline navigation, and template pre-population using tools like SUSIE and ComplianceAuthor AI.
In RA, it improves dossier completeness, automates eCTD publishing, and enhances compliance through predictive analytics.
Case studies from leading pharmaceutical companies demonstrate practical implementations, while challenges such as data quality, regulatory validation, ethical issues, talent shortages, high costing, and integration with legal and IT system are addressed.
Future perspectives emphasize AI’s potential for providing strategic recommendations, global fostering harmonization, and advancing personalized medicine, underscoring the need for interdisciplinary collaboration to maximize its impact on drug development and patient safety.
Introduction
The rapid advancement of ÁI is reshaping various sectors, with the pharmaceutical industry standing at the forefront of this transformation. In the pharmaceutical domain, AI’s transformative potential is evident in CMC – ensuring drug safety, efficacy, and quality – and Regulatory Affairs, which navigates compliance for market approval. By automating documentation, enhancing data analysis, and predicting compliance risks, AI streamlines processes like Module 3 CTD preparation and eCTD publishing, fostering efficiency and innovation. Despite benefits such as personalized medicine and global harmonization, challenges including data biases, ethical dilemmas, and regulatory validation must be addressed. This article examines AI’s applications, case studies, risks, and prospects in CMC and RA, highlighting its role in accelerating drug development while emphasizing responsible integration.
Artificial intelligence
Introduction to AI
AI can be categorized in different ways. From chatbots to super-robots, here are the types of AI to know and where the tech’s headed next.
If you’ve ever used Amazon’s Alexa, Apple’s Face Identification or interacted with a chatbot, you’ve interacted with AI.
There are many ongoing AI developments, most of which are divided into different types. These classifications reveal more of a storyline than a taxonomy, one that can tell us how far AI has come, where it’s going and what the future holds.
These are the seven main types of AI to know, and what we can expect from the technology.
7 Types of AI
- Narrow AI
- Artificial General Intelligence
- Artificial Superintelligence
- Reactive Machine AI
- Limited Memory AI
- Theory of Mind AI
- Self-Aware AI
One Liner for each AI type
- Narrow AI: AI designed to complete very specific actions; unable to independently learn.
- Artificial General Intelligence: AI designed to learn, think and perform at similar levels to humans.
- Artificial Superintelligence: AI able to surpass the knowledge and capabilities of humans.
- Reactive Machine AI: AI capable of responding to external stimuli in real time; unable to build memory or store information for the future.
- Limited Memory AI: AI that can store knowledge and use it to learn and train for future tasks.
- Theory of Mind AI: AI that can sense and respond to human emotions, plus perform the tasks of limited memory machines.
- Self-Aware AI: AI that can recognize others’ emotions, plus has a sense of self and human-level intelligence; the final stage of AI.
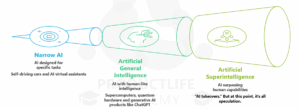
Capability-Based Types of AI
Based on how they learn and how far they can apply their knowledge, all AI can be broken down into three capability types: Narrow AI, Artificial General Intelligence and Artificial Superintelligence.
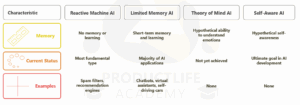
Functionality-Based Types of AI
Functionality is focused on how an AI applies its learning capabilities to process data, respond to stimuli and interact with its environment. As such, AI can be sorted into four functionality types.
In pharmaceutical regulatory affairs, Narrow AI acts as the umbrella for all current technologies, including machine learning for drug discovery optimization and natural language processing for efficient adverse event reporting and compliance. This task-specific AI boosts precision in submissions, data analysis, and risk management under global guidelines.
Now, the industry is shifting to Generative AI, a subset that creates new content like synthetic clinical trials, regulatory documents, or molecular predictions. This evolution accelerates innovation and market entry but requires strict oversight for ethics, data accuracy, and bias reduction to protect patients while advancing healthcare. Let’s explore further about Generative AI in details.
Generative AI:
Introduction to Generative AI
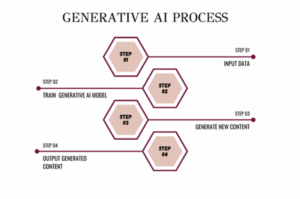
Generative AI: Can generate new data from the given input. These models are trained on datasets of examples and can generate new, previously unseen content.
What is Generative AI?
- Generative AI, simply put, is a class of AI models that have been developed to generate new data based on a given input. This could include various forms of content such as text, images, audio, and even video. These models are trained on datasets containing numerous examples and have the remarkable ability to generate new content that has not been seen before.
- Generative AI models are also known as foundation models which are pre-trained on comprehensive datasets that serve as examples for various types of content.
- They are powered by their ability to learn from extensive amounts of data
Features and Capabilities:
- Generate creative texts such as stories, poems or jokes
- Perform translations between different languages
- Explain complex concepts
- Provide detailed information on variety of subjects
Benefits of Generative AI
Generative AI offers many possibilities. It can positively impact operations, customer experience, decision-making, and overall efficiency. Let’s explore where it can achieve impressive results. Still, it’s important to note that while it can contribute to benefits, it might not solely drive all outcomes.
1. Enhanced Customer Experience:
- Provides better and personalized recommendations, helps in product selection, and offers virtual customer support to increase satisfaction, loyalty, and business growth.
2. Improved Decision Making:
- Analyses large volume of data, predicts market trends, and provides data-driven recommendations for informed choices and staying ahead in the competition.
3. Refine Operations:
- Automates and optimizes processes like production scheduling, supply chain logistics, and inventory management for improved efficiency, cost savings, and faster turnaround.
4. Cybersecurity:
- To provide a secure digital environment, it can detect and mitigate cyber threats, identify anomalies, analyze network traffic, and proactively respond to security breaches.
5. Employee Productivity:
- Helps in automating repetitive tasks, provide intelligent problem-solving assistance, and frees up time for value-added activities.
6. Competitive analysis and Market Research:
- Analyze data from available online sources, customer reviews, and social media to understand preferences, recognize market opportunities and maintain a competitive spirit.
7. Demand Prediction:
- Forecast demand patterns through analysis of market trends, historical data, and customer behavior to fine tune inventory, reduce stock outs, and create a robust supply management system.
Ethical Concerns and Associated Risks
Ethical Concerns
Generative AI is an emerging field that faces multiple ethical challenges.
1. Fair:
- Generative AI models can carry over data bias to outputs – incorrect data, biased sampling, and biased stereotypes in training data can lead to biased output.
2. Socially Beneficial:
- Applying Generative AI without addressing model bias can perpetuate existing biases and reinforce inequalities.
3. Explainable and transparent:
- Models can hallucinate and generate confident but false answers; the input-output relationship is hard to explain and not always transparent due to complexity.
4. Accountable:
- Models can be trained using data and output data irrespective of IP rights. There are ongoing debates on the legal framework and how to properly regulate Generative AI.
Current risks
Although a powerful tool, Generative AI is a novel and rapidly evolving solution that carries risks in its use – both with data input and output.
Data input risks
1. Training Data Issues:
- Inaccurate, biased, prohibited, and/or wrong data can be used to train the models. Models also lack real-time data.
2. Intellectual Property:
- Proprietary data is used to train the models, raising questions about copyright infringement.
3. Exposure of Sharing Information:
- Company proprietary data and/or personally identifiable information (e.g., patient privacy data) could be extracted from models via prompt engineering or more sophisticated abuse or attacks.
Disclosure of proprietary information could be argued as being a waiver of privilege.
4. Breach of Contract:
- Using third-party confidential information in a model could constitute breach of a third-party contract (e.g., a confidentiality agreement).
Data Output risks
1. ‘Hallucinations’ & False Answers:
- Data may look real but be fake or inaccurate. Examples are the “Deep fakes” cases, consisting of fake content in the requested style.
2. Fraud and Abuse:
- Models may create false reviews, false papers, spam, and/or phishing attacks.
3. Lack of Transparency and Explainability in the Data:
- It is difficult to check why a model reached a specific conclusion.
4. IP Protection & Infringement:
- Data output can create questions regarding inventorship/ownership of an AI-generated idea. (Note: Most countries do not allow AI to be an inventor on a patent.)
- Data output may include protected information or ideas that may trigger undesirable licensing obligations for open-source modules.
Role of Artificial Intelligence in CMC and Regulatory Affairs
AI is transforming pharmaceutical CMC and Regulatory Affairs by improving efficiency, accuracy, and compliance.
AI accelerates drug development, optimizes manufacturing, and automates regulatory document preparation. Despite challenges like data quality and regulatory acceptance, AI holds promise for personalized medicine and global regulatory harmonization.
Chemistry, Manufacturing, and Controls (CMC)
CMC is a critical component of pharmaceutical development, encompassing the chemistry, manufacturing processes, and quality controls of drug substances and products. It ensures that the drug is safe, effective, and of high quality.
Regulatory Affairs (RA)
Regulatory Affairs, on the other hand, involves ensuring that pharmaceutical products comply with all applicable regulations and laws, facilitating the approval and marketing of these products.
The integration of AI in CMC and RA offers numerous benefits, including the automation of routine tasks, improved data analysis, and enhanced decision-making capabilities.
AI in CMC:
In CMC, AI-driven tools are revolutionizing the way documentation is prepared and maintained, particularly for Module 3 of the CTD, which is essential for regulatory submissions. Module 3 provides detailed information on the quality of the drug substance and product, including manufacturing processes, characterization, and stability data.
1. Automating Module 3 Preparation
AI can significantly streamline the preparation of Module 3 by automating data extraction from various sources, such as laboratory reports and manufacturing records, and organizing this data into the required format. For instance, tools like SUSIE (Pharmaceutical CMC ontology-based information extraction) utilize machine learning to extract relevant information from unstructured text in pharmaceutical documents, facilitating the creation of comprehensive and compliant documentation.
Moreover, platforms like Compliance Author AI employ structured content management to ensure accuracy and consistency, reducing manual effort and enhancing the quality of CMC documentation.
2. Developing Response Documents and Briefing Books
AI accelerates the creation of high-quality response documents and briefing books by quickly gathering and summarizing pertinent information from large datasets. Natural language processing can identify key information from prior submissions or regulatory feedback, ensuring comprehensive and targeted responses to health authority queries. This capability minimizes errors and enhances the quality of interactions with regulators.
3. Navigating ICH Quality Guidelines
Based on the ICH guidelines, the AI-powered systems can interpret and apply these guidelines to ensure that regulatory submissions are aligned with the latest requirements. For example, AI can automate risk assessments, identifying potential quality issues based on historical data.
Tools like CMC AI search prescribing information and internal reports to populate templates, ensuring adherence to ICH guidelines.
4. Prepopulating Document Templates
AI enhances efficiency by prepopulating Module 3 templates with data extracted from existing documents or databases. Natural language generation tools can fill standard sections, leaving only unique information for manual input. This reduces preparation time and ensures consistency across submissions, supporting both development and lifecycle management phases.
AI in Regulatory Affairs (RA)
In Regulatory Affairs, AI-powered solutions are enhancing the completeness and consistency of regulatory dossiers, ensuring they meet global requirements. AI can analyse vast amounts of regulatory data, identify patterns, and predict potential compliance issues, thereby improving the accuracy of submissions.
1. Improving Dossier Completeness and Consistency
AI analyzes regulatory dossiers to identify missing information or inconsistencies, ensuring completeness. Machine learning models predict potential compliance issues by comparing current submissions against historical data. Regulatory Intelligence tools provide real-time access to regulatory updates and insights from national authorities worldwide, enabling companies to align dossiers with global standards.
2. Automating eCTD Publishing
The eCTD is the standard format for regulatory submissions. AI automates eCTD assembly, ensuring correct metadata and formatting. For example, a pharmaceutical company developed a regulatory intelligence assistant using natural language processing and large language models to provide dynamic insights and risk categorization, reducing submission errors and accelerating timelines.
3. Enhancing Compliance
AI supports compliance by monitoring regulatory changes and analysing submission data. Predictive analytics identify trends, helping companies anticipate regulatory shifts. This proactive approach minimizes non-compliance risks, as highlighted in the FDA’s initiatives to develop risk-based regulatory frameworks for AI.
AI in Post-Market Surveillance:
AI can rapidly analyse large volumes of real-world data-like electronic health records, social media, and registries-to detect emerging safety signals. This enables earlier identification of potential risks, allowing Regulatory Affairs teams to act proactively and support timely regulatory decisions. For example, Aetion Evidence Platform is used by pharma and regulators (including the FDA) to generate real-world evidence for post-market safety and effectiveness using AI-driven analytics.
AI in Pharmacovigilance:
Using machine learning and natural language processing, AI can automatically process and prioritize adverse event reports. This improves the efficiency, accuracy, and consistency of safety monitoring, directly supporting risk management, labelling updates, and compliance with global regulatory requirements. For example, AE Tracker which uses natural language processing and AI to capture and analyse adverse events from digital and social media sources.
Case Studies and Examples
Several pharmaceutical companies and technology providers have successfully implemented AI solutions in CMC and RA.
1. Regulatory Intelligence Assistant
A leading pharmaceutical company utilized natural language processing and large language models to create a regulatory intelligence assistant. This tool provides team members with easy access to updated regulatory intelligence and risk categorization for substances of interest, enabling dynamic insights into various regulatory landscapes.
2. ComplianceAuthor AI
This platform supports CMC documentation by integrating AI with structured content management, ensuring compliance with regulatory standards and streamlining collaboration among pharmaceutical teams.
3. SUSIE
Designed for CMC, SUSIE employs machine learning to extract CMC-specific information from publicly available prescribing information and internal development reports, streamlining the process of populating IND/IMPD and NDA/MAA templates.
4. Regulatory Intelligence
This tool provides instant access to global regulatory updates, supporting RA teams in maintaining compliance and optimizing submission strategies.
Challenges and Considerations
While the benefits of AI in CMC and RA are substantial, there are challenges and considerations that must be addressed.
1. Ethical Considerations
Data privacy, algorithmic transparency, and accountability are critical.
2. Regulatory Validation
Validating AI tools for regulatory acceptance is complex. The FDA’s draft guidance (2025) addresses AI’s role in supporting regulatory decisions, highlighting the need for risk-based validation.
3. Data Quality and Reliability
AI relies on high-quality data. Inconsistent or incomplete datasets can lead to errors, necessitating robust data management systems.
4. Talent and Standards
A shortage of AI expertise and lack of standardized protocols hinder adoption. Interdisciplinary collaboration is essential to overcome these barriers.
5. High Implementation and Maintenance Costs
Deploying AI systems requires significant upfront investment. For smaller companies, this can be a major barrier. Ongoing costs for system updates, maintenance, and compliance monitoring further add to the burden.
6. Integration with Legal and Legacy IT Systems
Pharma companies often rely on complex; legacy IT infrastructures tied closely to legal and regulatory processes. Integrating new AI tools into these systems is rarely straightforward. Ensuring compliance with data integrity standards, GxP, and global regulations adds another layer of complexity, especially when legal accountability for AI-driven decisions is still evolving.
Future Perspectives
As AI technology continues to evolve, its applications in CMC and RA are expected to expand further. Future advancements may include more sophisticated AI models capable of providing strategic recommendations for regulatory pathways, optimizing drug development processes, and enhancing patient safety through advanced data analytics.
Collaboration between pharmaceutical companies, technology providers, and regulators will be crucial to establish standards, address ethical concerns, and maximize AI’s potential.
Conclusion
The integration of AI in CMC and Regulatory Affairs represents a paradigm shift in the pharmaceutical industry, offering unprecedented opportunities to enhance efficiency, accuracy, and compliance. By automating routine tasks, improving data analysis, and providing intelligent insights, AI is becoming an indispensable tool for modern regulatory strategies. As the industry continues to embrace innovation, AI will play a crucial role in driving efficiency across the product lifecycle and better addressing patient needs.
ABBREVIATIONS
| CMC | : | Chimica, produzione e controlli |
| AR | : | Affari normativi |
| intelligenza artificiale | : | Intelligenza artificiale |
| AE | : | Evento avverso |
| ICH | : | Consiglio internazionale per l’armonizzazione dei requisiti tecnici per i prodotti farmaceutici per uso umano |
| GxP | : | Buone pratiche x (ad esempio, GMP, GCP) |
| GMP | : | Buone pratiche di fabbricazione |
| GCP | : | Buona pratica clinica |
| SUSIE | : | Strumento di estrazione di informazioni basato sull’ontologia CMC farmaceutica |
| CTD | : | Documento tecnico comune |
| eCTD | : | Documento tecnico comune elettronico |
| Proprietà intellettuale | : | Proprietà intellettuale |
| FDA | : | Amministrazione per gli alimenti e i farmaci |
| IND | : | Nuovo farmaco sperimentale |
| Accordo di riservatezza | : | Domanda di autorizzazione per un nuovo farmaco |
| Impd | : | Dossier del medicinale sperimentale |
| MAA | : | Domanda di autorizzazione all’immissione in commercio |
This article was written by:
with the support of:
Bibliography
- https://builtin.com/artificial-intelligence/types-of-artificial-intelligence
- Vipul Mann, Shekhar Viswanath, Shankar Vaidyaraman, Jeya Balakrishnan, Venkat Venkatasubramanian, SUSIE: Estrazione di informazioni basata sull’ontologia CMC farmaceutica per lo sviluppo di farmaci mediante apprendimento automatico,
Computers & Chemical Engineering, Volume 179, 2023, 108446, ISSN 0098-1354, https://doi.org/10.1016/j.compchemeng.2023.108446. ( https://www.sciencedirect.com/science/article/pii/S0098135423003162 ) - Glemser Technologies. (2024). Eccellenza CMC basata sull’intelligenza artificiale con gestione dei contenuti strutturata, da
https://glemser.com/blog/ai-powered-structured-content-management-cmc-excellence/
- Sfruttare l’intelligenza artificiale per accelerare lo sviluppo di farmaci CMC normativi
https://www.linkedin.com/pulse/leveraging-ai-accelerate-regulatory-cmc-drug-edward-narke-swu2e
- Pronti per la trasformazione del CMC tramite l’intelligenza artificiale? Ecco come potrebbe accadere
https://www.qbdvision.com/ready-for-ai-to-transform-cmc-heres-how-it-could-happen/
- (2024). Come l’industria farmaceutica può migliorare la conformità normativa con la tecnologia basata sull’intelligenza artificiale, da
https://pharmaphorum.com/digital/how-pharma-can-improve-regulatory-compliance-ai-based-technology
- Abbattere le barriere: il futuro della CMC alimentato dall’intelligenza artificiale https://www.regulatoryrapporteur.org/artificial-intelligence/breaking-barriers-the-future-of-cmc-powered-by-ai/752.article
- Come l’innovazione digitale sta trasformando la CMC
https://www.pharmalex.com/thought-leadership/blogs/how-digital-innovation-is-transforming-cmc/
- https://www.ich.org/page/ich-guidelines
- La valutazione del rischio basata sull’intelligenza artificiale rivoluziona lo sviluppo dei prodotti farmaceutici https://www.qualitydigest.com/inside/fda-compliance-article/ai-powered-risk-assessment-revolutionizes-pharma-product-development
- Quadro di controllo e rischio dell’apprendimento automatico
- Prospettive normative per l’implementazione di AI/ML in ambienti GMP farmaceutici https://pmc.ncbi.nlm.nih.gov/articles/PMC12195787/
- Documento di discussione della FDA statunitense: Intelligenza artificiale nella produzione di farmaci file:///C:/Users/baps/Downloads/OPQ_ArtificialIntelligenceDiscussion_230222_Final.pdf
- Documento di riflessione dell’EMA sull’uso dell’intelligenza artificiale (IA) nel ciclo di vita dei medicinali
- 2 importanti cambiamenti nell’ICH Q9 che dovresti conoscere
https://zamann-pharma.com/2025/01/06/2-major-shifts-in-ich-q9-you-should-know/
- Dalla teoria alla pratica: passaggi per implementare con successo la gestione del rischio di qualità ICH Q9
- L’impatto continuo dell’intelligenza artificiale sulla manutenzione del ciclo di vita nel 2025 https://www.contractpharma.com/exclusives/the-continued-impact-of-ai-on-lifecycle-maintenance-in-2025/
- Intelligence normativa IQVIA
- Ruchika S. Patil, Samruddhi B. Kulkarni, Vinod L. Gaikwad, Intelligenza artificiale negli affari normativi farmaceutici, Drug Discovery Today, Volume 28, Numero 9, 2023,103700, ISSN 1359-6446,
Italiano: https://doi.org/10.1016/j.drudis.2023.103700. ( https://www.sciencedirect.com/science/article/pii/S1359644623002167 )
- Come l’intelligenza artificiale automatizza la creazione di documenti per l’invio di fascicoli normativi https://alphalifesci.com/blog/how-ai-automates-document-authoring-for-regulatory-dossier-submissions
- Semplifica la creazione di sezioni eCTD con Narrativa ®️ Navigator https://www.narrativa.com/ectd-automation/
- Gestione delle richieste di autorizzazione: superare le sfide con l’efficienza AI-First. https://www.freyafusion.com/solutions/submission-management
- Come l’intelligenza artificiale e l’automazione stanno trasformando la pubblicazione e l’invio di documenti normativi nelle scienze della vita https://www.freyrsolutions.com/blog/how-ai-and-automation-are-transforming-regulatory-publishing-and-submissions-in-life-sciences
- Come l’automazione supporta la pubblicazione normativa durante la transizione verso eCTD 4.0 https://www.iqvia.com/blogs/2022/09/how-automation-supports-regulatory-publishing-amid-the-transition-to-ectd-4-0
- Sfruttare l’automazione per la pubblicazione normativa nel passaggio a eCTD 4.0 https://www.ddismart.com/blog/leveraging-automation-for-regulatory-publishing-amid-the-shift-to-ectd-4-0/
- Intelligenza artificiale e automazione nelle richieste normative
https://www.linkedin.com/pulse/ai-automation-regulatory-submissions-ian-crone-fpfbe
- Navigazione tra le richieste di regolamentazione: intelligenza artificiale e automazione in eCTD https://aquilasolutions.us/news/navigating-regulatory-submissions-ai-and-automation-in-ectd
- Come l’automazione sta trasformando i processi di pubblicazione normativa https://www.ddismart.com/blog/how-automation-is-transforming-regulatory-publishing-processes/
- Intelligenza artificiale e automazione nelle operazioni di regolamentazione: il futuro delle presentazioni eCTD, nome dell’autore: Sharath Reddy Venna, Indo-Am. I. Pharm & Bio.Sc., 2023, vol. 21, numero 2, 2023, ISSN 2347-2251 iajpb.com
- Intelligenza artificiale per lo sviluppo dei farmaci
- Applicazione dell’intelligenza artificiale in un ambiente GMP/di produzione: un approccio industriale https://www.efpia.eu/media/vqmfjjmv/position-paper-application-of-ai-in-a-gmp-manufacturing-environment-sept2024.pdf
- “Intelligenza artificiale negli affari normativi farmaceutici: trasformare l’intelligence normativa, la conformità e la sicurezza dei farmaci.ˮ International Journal of Research Publication and Reviews, Vol (6), Numero (7), Luglio (2025), Pagina – 280-288, Sig.ra Sanika Dhananjay Sapate, Sig.ra Prajkta Balakshe, Dott. V. K. Redasani, Sig.ra Srushti Ramdas Shinde
- Fu L, Jia G, Liu Z, Pang X, Cui Y. Applicazioni e progressi dell’intelligenza artificiale nella regolamentazione dei farmaci: una prospettiva globale. Acta Pharm Sin B. 2025 gennaio;15(1):1-14. doi: 10.1016/j.apsb.2024.11.006. Epub 2024 novembre 13. PMID: 40041903; PMCID: PMC11873654.
- https://cmc-ai.com/services
Register to our news and events
Go to our Events to register
Go to our News to get insights
