
Q9(R1) Quality Risk Management
20 july 2023

Risk Management Principles and Their Importance
Risk management principles are important guidance for many industries, specifically pharmaceutical firms, to enhance the application of effective Quality Risk Management by industries and regulators. Quality risk management (QRM) is an overall and systematic process of minimising risks to product quality throughout its life cycle to optimise its benefit and balance the risk2. Risk is defined as the combination of the probability of occurrence of harm and the severity of that harm1.
The pharmaceutical industry must have quality tools & principles in place. It is mandatory to have a well-established quality risk management system as an integral part of an industry through which a potential risk can be well identified and mitigated at the early stages or managed on time.
An efficient quality risk management system shall ensure the delivery of a quality medicinal product to the consumers by proactively identifying and controlling potential quality issues during development, manufacturing, and distribution1.
What is Quality Risk Management
Quality Risk Management Principles help industries to deliver a quality medicinal product to consumers by providing a proactive way to identify and control potential quality issues during development, manufacturing, and distribution & throughout the product life cycle1.
Principles of Quality Risk Management
The basic principles of Quality risk management are listed below:
- Quality risk evaluation should be based on scientific knowledge, such as research & data, with the final goal of patient safety1.
- The higher the risk level, the stronger a QRM process should be. The effort, procedure, and documentation level should be proportional to risk 1.
- Quality risk management should be dynamic, iterative and responsive to change2.
- The potential for continual development and enhancement should be implanted in the quality risk management process2.
- Situations of supply delays impacting patient health are part of Quality Risk1.
Quality Risk Management Process1
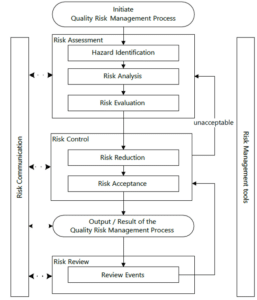
Quality risk management is a systematic process involving evaluation, control, prevention and review of risk to the quality of the drug product across the product life cycle. A flow chart which describes the steps involved in the quality risk management process is outlined below (Figure 1).
Figure 1: Overview of a typical quality risk management process
Risk Management Tools
The industries can utilise a variety of tools1 listed below for quality risk management, either alone or in combination, based on the severity of the risk.
- Basic risk management methods (flowcharts, check sheets, Cause and Effect Diagrams (also called an Ishikawa diagram or fishbone diagram etc.)
- Failure Mode Effects Analysis (FMEA)
- Failure Mode, Effects and Criticality Analysis (FMECA)
- Fault Tree Analysis (FTA)
- Hazard Analysis and Critical Control Points (HACCP)
- Hazard Operability Analysis (HAZOP)
- Preliminary Hazard Analysis (PHA)
- Risk ranking and filtering
- Supporting statistical tools
Integration of Quality Risk Management into Industry and Regulatory Operations1
A well-developed quality risk management process needs to be efficiently integrated into the pharmaceutical industry through established documentation and standard operating procedures for its effective function during the needs. The effective integration of the quality risk management process into the quality systems of an industry helps in concluding practical decisions at times. Also, it builds confidence in companies’ quality systems for regulators.
Appropriate training and education of relevant personnel involved in the industry on Quality management process principles and their tools are essential for its effective implementation.
Review of the Principle of Quality Risk Management
The investigation of risk involves a scientific and practical approach. The quality risk management process consists of four major elements: risk assessment, risk control, risk review and communication. A well-established quality risk management process ensures that risks are adequately managed, eventually producing high-quality products.
PLG will be happy to help you if you are looking for any services related to Quality aspects of the pharmaceutical industry during your product life cycle journey.
References
- International Conference on Harmonization Guidance for Industry: Q9 Quality risk management Q9(R1). ICH; January 2023.
- International Journal of Pharmaceutical Sciences Review and Research Int. J. Pharm. Sci. Rev. Res., 44(1), May – June 2017; Article No. 34, Pages: 142-148.
Register to our news and events
Go to our Events to register
Go to our News to get insights
