
Introduction to the ASEAN Common Technical Dossier (ACTD) for Pharmaceutical Registration
11 july 2023

Introduction to the ASEAN Common Technical Dossier (ACTD) for Pharmaceutical Registration
The ASEAN Common Technical Dossier (ACTD) is a format for applications submitted to ASEAN regulatory authorities to register pharmaceuticals for human use. ASEAN countries such as Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines, Singapore, Thailand, and Viet Nam accept submissions in this format. A guideline agreed upon by the ASEAN countries describes how to compile applications in this format.
What is the difference between eCTD (ICH CTD) and ACTD?
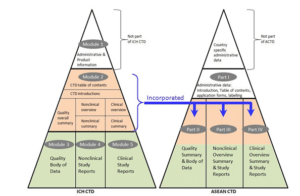
The ACTD consists of Parts I to IV, whereas ICH CTD has 5 Modules. Parts II to IV correspond to Modules 3 to 5, respectively. In ACTD, the summaries of the quality (Part II), nonclinical (Part III) and clinical (Part IV) are at the beginning of each part of the ACTD sections. In eCTD, there is a specific module 2 for these same summaries.
Unlike the eCTD, ACTD does not have an XML backbone. Instead, it has an overall Table of Content and section-specific Table of Content like we often see in the Non-eCTD Electronic Submission (NeeS) format.
How can we help with your ASEAN dossiers?
At Product Life Group (PLG), we have a dedicated team experienced in submitting regulatory applications globally. We can support you in converting/rewriting your existing dossiers (which could be in a national or eCTD template into an ACTD template and subsequently publish in ACTD format.
Register to our news and events
Go to our Events to register
Go to our News to get insights
