
IDMP: Support Data Governance in the Pharmaceutical Industry
16 november 2023

The benefits of IDMP throughout the pharmaceutical industry.
First of all, implementing IDMP standards has several benefits applied to different pharmaceutical industry departments:
- Pharmacovigilance
- Adverse event reports are based on a harmonized set of product definitions, improving the quality of data used for signal management and speeding up communication, decision-making, and actions.
- Regulatory Affairs
- Submissions use a consistent standard to capture and manage data, allowing information on medicinal products to be shared and re-used across different procedures and among various regulators.
- Clinical
- Stakeholders can access Clinical Trial data using agreed and well-supported standards, improving the assessment and scientific evaluation of medicines, communication, and transparency.
- Quality
- Inspections on manufacturing sites are based on accessible information, which streamlines inspections, particularly for urgent situations involving defects. Faster detection of falsified medicines can also be supported due to consistent data standards.
Consequently, Data Governance within R&D is becoming more and more popular within the industry:
- Data ownership, internal single source of truth
- Master Data Management aligned to common standards from Health Authorities used by several departments.
How will IDMP impact Data Governance?
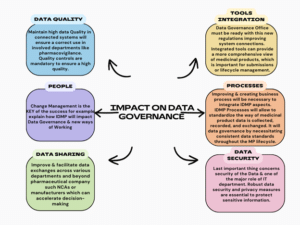
The importance of data management
In summary, IDMP requires rigorous data management for medicinal products. Effective data governance is crucial for ensuring regulatory compliance, data quality, and security while facilitating information exchange between pharmaceutical companies and competent authorities in the pharmaceutical industry. For example, in the EU, the new portals such as PLM Portal, SPOR, IRIS, and CTIS.
PLG’s support in your IDMP and data governance journey
For many years, ProductLife Group managed a significant number of projects in RIMS, IDMP, and regulatory projects. Moreover, ProductLife Group has a team combining multiple expertise addressing all stakes of a pharmaceutical company concerning IT and business sides, data governance, data centricity, process, program management, and change management to meet the objectives of IDMP Data Governance compliance.
Are you ready to tackle the IDMP challenge? Share your thoughts on its impact on data governance in the comments!
Join our experts on November 21st for our latest webinar “How can Smart Automation empower IDMP Readiness?”
Find more information on the webinar here.
Register here.
Register to our news and events
Go to our Events to register
Go to our News to get insights
