Extension of US-EU Dossier to ASEAN Countries from CMC Perspective
27 july 2023

Who are the ASEAN Countries, and what are they about?
The Association of Southeast Asian Nations (ASEAN) has ten member countries in Southeast Asia: Indonesia, Malaysia, Philippines, Singapore, Thailand, Brunei Darussalam, Vietnam, Laos, Myanmar and Cambodia. It was established in 1967 to promote the pharmaceutical market in ASEAN countries. These 10 ASEAN countries have different regulatory authorities for drug approval. It has diverse regulatory requirements for registering drug products. Though all the countries are harmonised, every country still differs in some local requirements such as administrative, quality, clinical and non-clinical documents; for example, in the present scenario, countries like Cambodia, Laos, Vietnam and Myanmar are looking to import generic drugs.
All ten countries seek economic development to improve competitiveness by eliminating trade barriers. Many ASEAN regulatory guidelines are harmonised with ICH and EU guidelines to remove these trade barriers. However, country-specific requirements persist. In this scenario, the pharmaceutical companies who wish to extend their marketing authorisation (MA) of regulated market products to ASEAN countries must understand such country-specific requirements for successful drug approval. The baseline dossier of Europe/US can be extended to ASEAN countries with some minor amendments. In this article, we will discuss a few peculiar requirements for ASEAN countries which should be considered while extending marketing authorisation.
Dossier format
The ASEAN region has developed ASEAN Common Technical Requirements (ACTRs), which provide guidelines for compiling drug dossiers and are comparable to Europe.
ACTD dossier format is accepted in ASEAN countries. However, CTD (ICH) is also acceptable in some countries. The ACTD and CTD Modules (parts) are detailed in the below triangles.
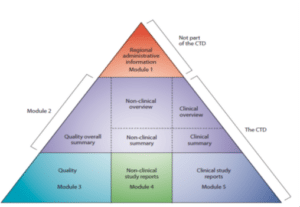
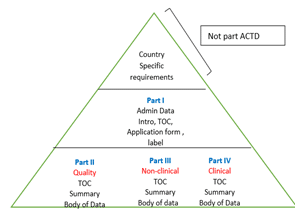
The above triangles show that the CTD is divided into Modules, whereas ACTD is divided into Parts. The detailed difference is tabulated below.
| CTD Section | Sections | ACTD Section | Sections | Remarks |
| Module 1 | It depends on the regional requirements | Part I | Section A: Introduction | Module 1 Part I Section C are administrative and regional |
| Section B: Complete ACTD Table of Content | ||||
| Section C Administrative documents | ||||
| Module 2
(Overall summary) |
2.1 Table of contents.
(Module 2-5) |
Part II
(Quality) |
Section A :
Table of contents |
|
| 2.2 Introduction | ||||
| 2.3 Quality overall summary | ||||
| 2.4 Non-clinical Overview | Section B:
Quality Overall Summary |
Part II Section B is equivalent to Module 3, 3.2 Body of data section.
|
||
| 2.5 Clinical Overview | ||||
| 2.6 Non-clinical Written and Tabulated Summaries | ||||
| 2.7 Clinical Summary | Section C:
Body of Data |
Part II Section C is equivalent to Module 3, 3.2 Body of data section.
|
||
| Module 3
(Quality Appendices and Regional) |
3.1 Table of Content | Part III
(Non-Clinical) |
Section A: Table of Contents | Part III is equivalent to Module 4
|
| 3.2.Body of Data | Section B: Non-clinical Overview | Part III Sections B &C are equivalent to Module 2 to 2.5 and 2.7 sections | ||
| Section C: Non-clinical Written and Tabulated Summaries | ||||
| 3.3 Literature references | Section D: Non-clinical Study Reports | |||
| Module 4
(Non-clinical Study Report) |
4.1 Table of Contents of Module 4 | Part IV
(Clinical Document) |
Section A: Table of Contents | Part IV is equivalent to Module 5 |
| 4.2 Study Reports | Section B: Clinical Overview | Part IV Sections B &C are equivalent to Module 2 to 2.4, 2.6 and 2.7 sections. | ||
| Section C: Clinical Written and Tabulated Summaries | ||||
| 4.3 Literature References | Section D: Tabular Listing of All Clinical Studies | |||
| Section E: Clinical Study Reports | ||||
| Section F: List of Key Literature References | ||||
| Module 5
(Clinical Study Report) |
5.1 Table of Contents of Module 5 | NA | NA | Module 5 is equivalent to Part IV of the ACTD section. |
| 5.2 Tabular Listing of All Clinical Studies | ||||
| 5.3 Clinical Study Reports | ||||
| 5.4 Literature Reference |
Chemistry Manufacturing Control (CMC) :
Among the 10 ASEAN countries, we will focus on four countries – Singapore, Malaysia, Thailand and the Philippines. Extending Europe or US dossier to these four countries seems similar, but simple copy-paste exercises will not work out; tapping into expertise can help avoid common mistakes. The comparison of the requirement of a few CMC documents is listed in the below table.
| CMC requirement | US | Europe | Singapore | Malaysia | Thailand | Philippines |
| Drug substance | ||||||
| DMF/ASMF/ CEP (Certificate of European Pharamacopeia) | DMF is recommended | ASMF/
CEP is recommended |
DMF/ASMF/CEP is recommended | DMF/ASMF/CEP is recommended | DMF/ASMF/CEP is recommended | DMF/ASMF/CEP is recommended |
| Acceptable pharmacopoeial grade | USP /Ph.Eur. is recommended | Ph.Eur. /USP. is recommended | USP/Ph.Eur | USP/Ph.Eur | USP/Ph.Eur | USP/Ph.Eur |
| Reference standard | USP /Ph.Eur. is recommended | Ph.Eur. /USP. is recommended. | USP/Ph.Eur | USP/Ph.Eur | USP/Ph.Eur | USP/Ph.Eur |
| Drug Product | ||||||
| Acceptable pharmacopoeial grade | Ph.Eur. /USP. is recommended | Ph.Eur. /USP. is recommended | USP/Ph.Eur | USP/Ph.Eur | USP/Ph.Eur | USP/Ph.Eur |
| Excipients | Ph.Eur. /USP. is recommended | Ph.Eur. /USP. is recommended | USP/Ph.Eur | USP/Ph.Eur | USP/Ph.Eur | USP/Ph.Eur |
| BMR (Batch Manufacturing Record) | Required | Not Required | Unexecuted BMR is Required | BE Batch BMR Required | BE Batch BMR Required | BE Batch BMR Required |
| Reference standard | Ph.Eur. /USP. is recommended | Ph.Eur. /USP. is recommended. | USP/Ph.Eur | USP/Ph.Eur | USP/Ph.Eur | USP/Ph.Eur |
| Stability | Zone II | Zone II | Zone IVb | Zone IVb | Zone IVb | Zone IVb |
| Product interchangeability (Bioequivalence) | ||||||
| Reference drug for BE | US RLD | EU Reference drug | Any country’s reference drug is acceptable if the manufacturing site address is similar to the Singapore reference drug. | Any country’s reference drug is acceptable if the manufacturing site address is similar to the Malaysian one. | Any country’s reference drug is acceptable if the manufacturing site address is similar to Thailand’s reference drug. | Any country’s reference drug is acceptable if the manufacturing site address is similar to the Philippines’ reference drug. |
| Biowaiver
(Reference drug used from a foreign country) |
Not acceptable | Not acceptable | Multimedia media dissolution against BE reference drug vs Singapore reference drug | Multimedia media dissolution against BE reference drug vs Malaysia reference drug | Multimedia media dissolution against BE reference drug vs Thailand reference drug | Multimedia media dissolution against BE reference drug vs Philippines reference drug |
| BE Studies | BE studies against US RLD performed at local and foreign sites are acceptable. | BE studies against EU RLD performed at local and foreign sites are acceptable. | BE studies against EU/US RLD performed at local and foreign sites are acceptable*. | BE studies against EU/US RLD performed at local and foreign sites are acceptable.* | Local BE for non-Onco products.
For Oncological products, BE studies against EU/US RLD performed at local and foreign sites are acceptable.* |
BE studies against EU/US RLD performed at local and foreign sites are acceptable.* |
*Manufacturing site address similarity needs to be proved.
@ Audit clearance certificate from the regulated market or Malaysian regulatory agency is mandatory
The CMC requirement comparison in the above table shows that US/EU dossier extension is feasible in ASEAN countries; however, a few additional data requirements must be considered.
Special requirements
There is a similar outline for all countries. Still, it varies from their regulatory aspects, such as the need for a letter of authorisation, site master file, patient information leaflet, bioequivalence and clinical requirement.
Malaysia has two types of applications new generic drugs and hybrid drug applications. Based on whether the drug is a new drug or a different form of active ingredient is used other than the reference drug (e.g., salt form, premix) or similar to the reference drug, the registration process is concluded. The requirement for hybrid registration is few distinct, so involving the expertise can help avoid common mistakes. There are specific requirements like raw data of analytical method validation, declarations for similarity of a foreign reference drug product and reference product of Malaysia, In-use stability data at initial and end of shelf life, a snapshot of BE reference product, and many 12 months stability data of finished product is required for submission.
For Vietnam, there are some definite requirements; few stamped quality documents are required from the drug product manufacturer and drug substance manufacturer. Compendial monographs are submitted for excipients and drug substances if claimed, and Pharmacopeial versions must be declared. Raw data of analytical methods are required, and a Snapshot of BE reference product is required and many. In very few cases, 12 months of stability data of the finished product is required for submission.
Based on current guidelines for Thailand, we need to submit a pre-submission application and the actual submission in CTD format. In the quality section, precise requirements like chromatograms for drug product and drug substance, compendial monographs with a version of pharmacopoeia and page number need to be declared; monographs need to be enclosed and a snapshot of BE reference product. In very few cases, 12 months of stability data of the finished product is required for submission.
Myanmar exhibits a high import rate compared to other countries and has some country-specific guidelines for market authorisation. The sample requirement is the major one. We require the complete analysis of drug products performed in the authorised labs for Oncological products. Samples are required for drug registration and variations. Twenty-four months of stability data of the finished product is required for submission.
Life cycle management
When a Pharmaceutical company extends their EU/US dossier to ASEAN countries, any changes proposed during life cycle management must also be adopted for ASEAN countries. So it is important to understand similarities and differences in life cycle management for EU/US and ASEAN countries. ASEAN variation guidelines only apply to human-made pharmaceutical products, excluding biologics. As per ASEAN variation guidelines, variations are classified into two broad categories – major and minor.
- Major Variation (MaV) significantly impacts finished product quality, safety and efficacy.
- The Minor Variation (MiV) category is subdivided into Notification and Prior approval, depending upon the impact of change on finished product quality, safety and efficacy.
The comparison of ASEAN, US and EU variation classification
| ASEAN VARIATION CLASSIFICATION IN SYNC WITH EU AND US | |||
| Types of Variations | ASEAN Classification | EU Classification | US Classification |
| Minor (Notification) | MiV – N | Type IA –
Do and Tell |
CBE – 0 |
| Minor (Prior Approval) | MiV – PA | Type IB | CBE – 30 |
| Major (Prior Approval | MaV | Type II | Prior Approval Supplement (PAS) |
From the above comparative table, the variation requirements seem similar for US/EU and ASEAN. However, due to the agency’s understanding and evaluation, the variation classification for the same change will differ for ASEAN, US and EU. Such differences necessitate experts to pitch in for the correct evaluation of variation. PLG has a team of experts who can direct you towards the right evaluation of post-approval changes for ASEAN, EU and US.
For example: For Malaysia, Thailand and Vietnam, Excipients vendors are not committed, while for Europe and the US, it is a commitment.
Changes in BMR are not a commitment.
PLG’s Role in ACTD and how we can support you
The ASEAN region is emerging in the pharmaceutical marketplace. We at PLG provide the services for dossier gaps analysis, writing and review of the dossiers to be extended from EA-EU and the US. Our experts ease the process for pharmaceutical companies looking to penetrate these regions more effectively. Alongside our strategic advice, we offer tactical resources and expertise to address questions raised by health authorities and regulatory bodies.
Register to our news and events
Go to our Events to register
Go to our News to get insights
