
An Overview of the Pharmaceutical Regulations in Tunisia
13 january 2023

Tunisia is the first African country to establish a pharmaceutical industry based on its well-structured regulation, supporting pharmaceutical companies to expand their market size, which was estimated at USD 1.3 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of 12.9% from 2021 to 2028.(1)
The Pharmaceutical regulations are structured around different public bodies involved in various pharmaceutical functions from product registration, site inspection, control, manufacturing, clinical trials, promotion and supply to dispensing medicines to patients.
The Directorate of Pharmacy and Medicine (DPM)
The Directorate of Pharmacy and Medicine is a technical administrative body of the Ministry of Health. The DPM manages all administrative aspects related to pharmacies, medicine and relevant activities such as Marketing Authorisation Approval (AMM) for pharmaceuticals for human and veterinary use and Authorisation for Market Consumerization (AMC) for imported medicines, medical devices and food supplements, etc. It coordinates the activities of the national system for quality assurance in the pharmaceutical industry. The DPM plays a supervisory role for public bodies where medicinal products are concerned. Since 1998, it has been operating as a WHO Collaborating Centre for medicinal product registration and pharmaceutical regulations.(2) The DPM receives trainees from approximately twenty different countries who visit to benefit from Tunisian expertise in regulation. The DPM also sends its experts to around thirty countries to assist them and provide training relating to the Tunisian digital registration system.(3)
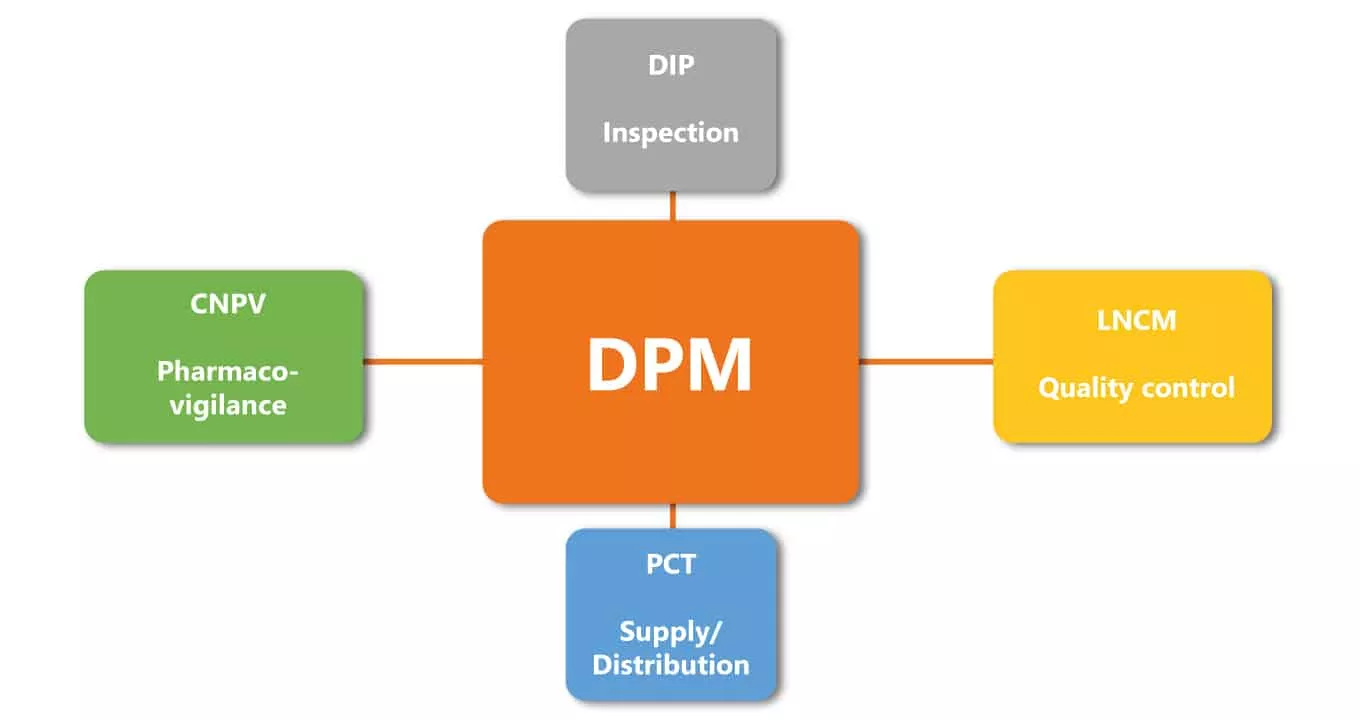
The National Pharmaceutical Control Laboratory (LNCM)
The main missions of the national pharmaceutical control laboratory, under the supervision of the Ministry of Health, are to carry out analyses and evaluation studies of medication documentation and products for therapeutic use, personal hygiene, cosmetics and any other related products intended for human or veterinary use. The laboratory also manages the quality control of these drugs and healthcare products before, during and after their market release. It guarantees the quality of pharmaceutical products intended for the local market or export.(4)
The Pharmaceutical Inspection Department (DIP)
The Pharmaceutical Inspection Department is an independent directorate within the Ministry of Health. It ensures compliance with and adherence to the laws and regulations applicable to the pharmaceutical professions and pharmaceutical products for human and veterinary use. It facilitates the required inspection, assessment and investigation operations along with the management of all inspections concerning manufacturers, wholesalers-distributors, pharmacies, health establishments with a pharmacy service and owners of medication supply stocks.(3)
National Center for Pharmacovigilance (CNPV)
The National Pharmacovigilance Center was created in 1984 under the supervision of the Ministry of Health. The pharmacovigilance system guarantees the continual monitoring of Tunisian-marketed drugs and their efficacy in terms of quality and safety.
The CNPV has an ongoing collaboration with international pharmacovigilance procedures and in particular with the WHO Collaborating Center for International Drug Monitoring in Uppsala, Sweden. This has facilitated Tunisia’s membership since August 1993 as the fortieth member country of the WHO international pharmacovigilance program.
In addition to its responsibilities related to pharmacovigilance, the CNPV has set up its bioequivalence centre which is open to both local and foreign pharmaceutical industries.(5)
Tunisia’s Central Pharmacy (PCT)
The Central Pharmacy, under the supervision of the Ministry of Health, plays a major role due to its exclusivity on the import of all medication, vaccines, serum and related products It also has sole responsibility for supplying these products to public health facilities. This approach is a feature of the Tunisian state strategy. The PCT can thus help control drug purchasing costs and ensure their quality. The PCT also manages a strategic medication reserve.(6)
Registration of medicinal products in Tunisia
No medicinal product in Tunisia can be provided, either free of charge or against payment, without receiving a Marketing Authorisation from the Ministry of Health. This Marketing Authorisation is issued for 5 years and renewable for an additional 5 years. This requirement is formalised in the act n°85-91 of 22nd November 1985, regulating the manufacture and registration of medicinal products intended for human use in Tunisia.
The registration of pharmaceutical drugs in Tunisia is regulated by the Medicinal Products Registration Guide, published on 17th February 2016. This guide describes the registration procedure for drug products for Human and Veterinary use. The provisions related to the registration of specific medicinal products such as biosimilars, homoeopathic medicinal products, radiopharmaceuticals and herbal medicinal products are provided in specific documents.
The first edition of this document published in 2004 has been reviewed and updated to meet the evolution of the activity at national and international levels.
The guide deals with all requirements for registration of the drug product, in particular:
- for the content of the registration dossier: administrative and CTD modules.
- for post-marketing applications (variations, renewals, line extensions etc.)
The application dossier includes all the modules to be submitted, related to the application in compliance with ICH requirements in the French or English language.
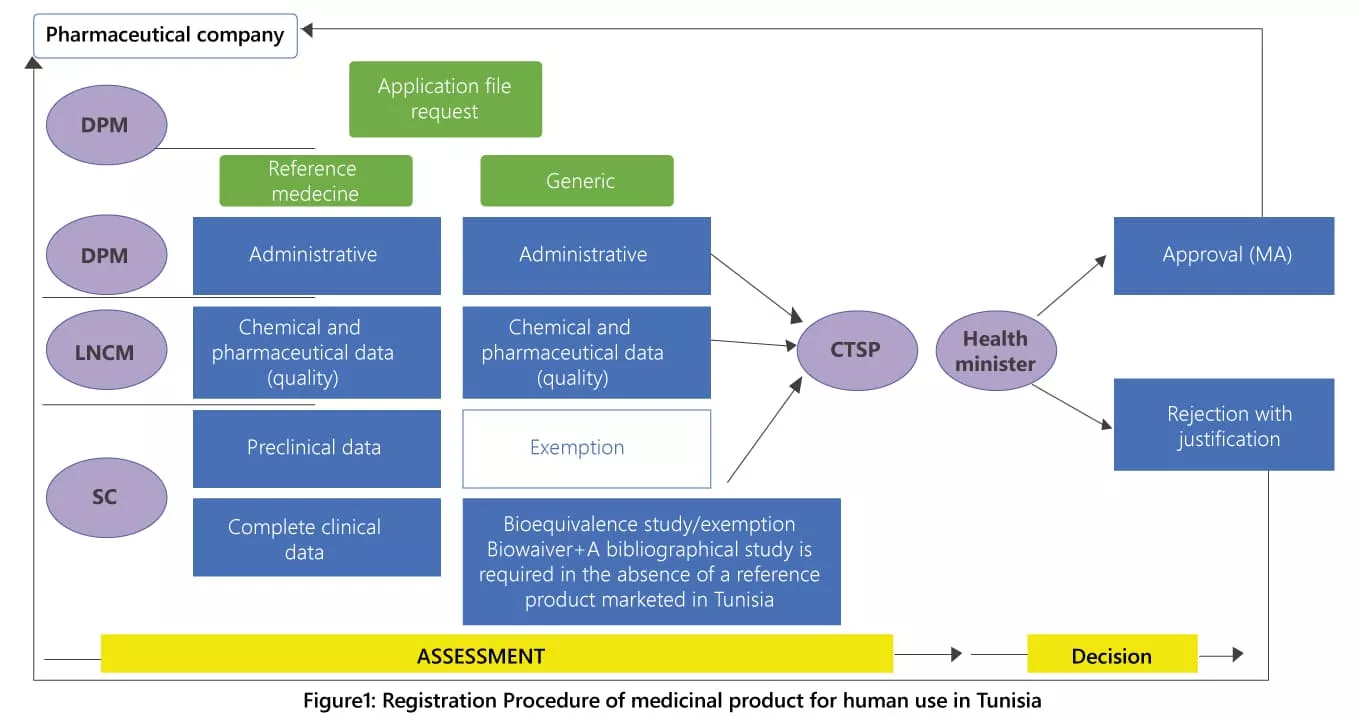
The concerned steps of the registration procedure for medicinal products, generics or reference products, intended for human use can be summarized below:
- Appointment Booking: to book via the DPM website a face-to-face appointment with the DPM
- Submission of the file: the applicant provides the dossier to the DPM for assessment and evaluation of the technical file and administrative documentation.
- Clinical and preclinical data assessment: Up to seventeen Specialized Committees (depending on the therapeutic class of the drug product) assess the files, taking into account the therapeutic benefit and the undesirable effects observed as well as the cost-effectiveness.
A calendar with planned meetings of the specialised committees is published on the DPM website every year, to enable the applicant laboratories to submit their files within the applicable time frames.
The files of the generic applications are exempted from this step except for the case where the reference product is not registered in Tunisia.
- Data quality assessment of medicinal products: The assessment of the pharmaceutical and chemical dossiers, as well as the analytical and microbial control are carried out by the LNCM. The decision of the LNCM is communicated to the applicant by the DPM.
- Assessment of the Marketing Authorisation application by the Technical Committee for Pharmaceutical Specialties (CTSP): The CTSP studies the conclusions reached by the specialized committees and the LNCM to either approve, reject or ask for further scientific or medical clarification.
- Marketing Authorisation Grant: The Marketing Authorisation is issued by the CTSP for 5 years. The applicant informs the Tunisian Ministry of Health of the effective date of entry into the market (date of first customer delivery) of the medicinal product by sending a notification letter to the DPM and by providing a packaging mock-up.(2)
- The internal guidelines of the CTSP are available on the website of the DPM (dpm.tn).
Having more than 20 years in delivering regulatory compliance services for the safe and effective use of medical solutions, ProductLife Group can support you in Tunisia in managing all your principal registration, clinical and CMC activities related to the Tunisian regulation requirements.
References
- https://www.grandviewresearch.com/industry-analysis/tunisia-pharmaceutical-market
- Pharmacy and Drug Directorate – Ministry Of Health – Tunisia available from: http://www.dpm.tn/images/pdf/guide_dpm_en.pdf
- THE HEALTH CARE SECTOR IN TUNISIA / PHARMACEUTICAL EXPERTISE https://tunisiecapitalesante.tn/en/wp-content/uploads/sites/2/2021/03/BROCHURE-TCS-EN-DAS-2.pdf
- http://www.lncm.tn/
- http://www.pharmacovigilance.rns.tn/
- http://www.phct.com.tn/en
Register to our news and events
Go to our Events to register
Go to our News to get insights
