
Strategic Considerations to Advance N-of-1 and N-of-Few RNA Therapies
09 december 2025

In the United States, the U.S. Food and Drug Administration (FDA) has formalized expectations for individualized antisense oligonucleotides (ASOs) under single-patient Investigational New Drug applications (INDs), creating a structured path for case-by-case development. In contrast, the European Union has no N-of-1-specific guidance yet, but there are efforts advancing through class-level oligonucleotide guidance and collaborative initiatives.
This dynamic propelled my collaborative research and my discussion at the recent European Congress on Rare Diseases and Orphan Drugs, titled, “Toward an Extensible Regulatory Framework for N-of-1 to N-of-Few Personalized RNA Therapy Design.”
While I addressed critical challenges across international regulatory bodies, risk management, and sustainability of individualized therapy programs, I also proposed an extensible framework for N-of-1 and N-of-Few RNA therapy designs with a conceptual roadmap for ultra- or nano-rare diseases that lack established therapies. With approximately 7,000 rare diseases identified, about 95% lack authorized treatments, highlighting a significant unmet medical need.
These diseases present unique challenges, as patients may represent the sole individual or a small group worldwide with a specific mutation, necessitating personalized approaches to treatment development, validation, and approval.
While progress is promising, the regulatory landscape remains nascent, raising challenges in ensuring safety and sustainability. But there is potential for improvements, lending itself towards a revised framework for these therapies, and the best way to evolve is through knowledge sharing and collaboration.
Regulatory Landscape Challenges
N-of-1 or bespoke therapies are tailored to the unique genetic characteristics of individual patients. Initially designed for a single patient, these therapies can also extend to small populations (N-of-Few) where various mutations within the same gene produce comparable effects on the gene product.
This adaptable strategy enables personalized medicine, taking account of the unique genetic and molecular makeup of each patient, facilitating the development of targeted solutions for a wide range of diseases while minimizing side effects and optimizing outcomes.
But this strategy is at odds with the current regulatory landscape. I argue that a more structured framework is necessary to maximize the potential of this approach while also ensuring safety, efficacy, and patient welfare.
While terms like “N-of-1,” “N-of-Few,” or “N-of-1 +” lack universal regulatory definitions, they generally describe approaches focusing on treating individual patients or small groups of patients rather than large populations and are commonly applied in personalized medicine.
Because of the highly personalized nature of the treatment, producing a standard clinical trial design is impractical due to the small sample sizes, creating unique challenges in achieving statistically significant results that are required for regulatory approval.
These challenges are further compounded by the need for individualized clinical outcome assessments and natural history data tailored to the patient’s unique genotype-phenotype, as well as a lack of harmonized standards across regulatory agencies (i.e., FDA and EMA), leading to variations in standards.
Collaborative Efforts in Advancing N-of-1 and N-of-Few Treatments
Because regulatory approaches to N-of-1 therapies remain unaligned across countries—and many jurisdictions lack N-of-1–specific pathways—it is essential to coordinate efforts and share knowledge across communities. Specifically, in Europe, the absence of a dedicated regulatory framework and authorization route for N-of-1 therapies has helped catalyze initiatives, like the European 1 Mutation 1 Medicine (1M1M), and global collaborations like the N1 Collaborative, aiming to facilitate communication, exchange expertise, and share data across borders.
Additional opportunities observed:
- Academic medical centers can also seek regulatory guidance from organizations like the EMA’s Innovation Task Force (ITF) to navigate regulatory hurdles
- Engage early (and often) with regulators to align scientific advice and best practices throughout the entire development process
Strategic Future Directions
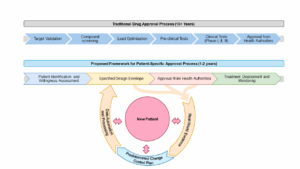
To tackle these complexities, I proposed, as noted during my presentation and in our research paper, a structured framework encompassing multidimensional parameters, leveraging real-world evidence and Artificial intelligence (AI)-driven approaches. See Figure 1.
Figure 1
 +
+
Drawing inspiration from established norms in the medical device industry, my team and I (in our research) propose a comprehensive framework built around a specified design envelope defining validated boundaries (chemistry, analytics, release specifications, and other parameters) within which a product can adapt safely for the range and type of N-of-1 and N-of-Few therapies developers seek approval to produce. Along this envelope, the Predetermined Change Control Plan (PCCP) concept, adapted from AI/ML change management, was introduced. The PCCP allows for predefined changes to be made within an approved design envelope, avoiding the need for a new marketing submission for each modification, provided these changes remain within the pre-approved scope of the plan. This approach enables a structured and efficient pathway from N-of-1 and N-of-Few treatments, and from N-of-1 treatments to highly related N-of-1 treatments for similar conditions.
This framework is sufficiently flexible to accommodate the unique attributes of RNA-based therapies. Additionally, AI algorithms assist in optimizing parameters within the design envelope. More broadly, AI supports patient identification, process optimization, real-time safety monitoring, and continuous learning from real-world evidence.
For example, AI could expedite the development of antisense oligonucleotides (ASOs) for N-of-1 and N-of-Few by analyzing patient data to pinpoint individuals with targetable genetic diseases. ASOs represent versatile tools for precisely modulating gene expression at the RNA level. In addition, AI may aid in swiftly identifying promising target sites and predicting optimal positioning for splice-shifting oligonucleotides, thereby reducing the number of compounds needing screening and accelerating the discovery of new ASOs.
However, further investigation into the intricacies of this framework, particularly around how PCCP-like approaches can be operationalized for RNA therapeutics, will be essential, forming the focus of forthcoming research endeavors and stakeholder validation.
Recent Complimentary Research
Aligned with our proposed framework, key contributions have been published since our research, as I noted during the presentation, including:
- From roadmap to a sustainable end-to-end individualized therapy pathway by Jonker at al., published in March 2025
- Regulatory sandboxes: A new frontier for rare disease therapies by Zanello et. Al., published in October 2025
The first paper listed above, From roadmap to a sustainable end-to-end individualized therapy pathway, provides the following complementary contributions:
- Expands the International Rare Diseases Research Consortium (IRDiRC) roadmap from conceptual framework to practical implementation pathway
- Addresses critical gap: distinguishes N-of-1 as innovative practice by way of experimental interventions to benefit patients, which sit between standard clinical care and traditional research and therefore face distinct regulatory and oversight issues
- Proposes dedicated global registry modeled after European Society for Blood and Marrow Transplantation (EBMT)
- Introduces subscription payment model and pay-for-performance models as sustainable funding mechanisms
- Highlights the need for international alignment on the definition of platform technology.
The second paper listed above, Regulatory sandboxes: A new frontier for rare disease therapies, proposes regulatory sandboxes – controlled regulatory environments allowing innovators to test novel products under regulatory supervision with predefined rules.
There are global examples already operating, including:
- Japan: AI/blockchain in healthcare
- China Hainan: RWE data sandbox for foreign drugs
- Health Canada: Foreign review reliance and COVID adaptations
- European Union: AI Act sandboxes; pharma legislation revision includes the concept
- European Innovative Health Initiative: Launched call for projects modeling regulatory sandboxes for breakthrough innovation
The key characteristics of these sandboxes include temporary, time-limited participation, regulatory flexibility allowing deviations from standard pathways, and structured collaboration between regulators and developers, working towards enabling clear rules for transition from sandbox to formal approval processes essential.
Taken together:
- Our Design Envelope + PCCP-like model defines what can change and how to govern it technically;
- The regulatory sandbox concept offers where to test new approaches under supervision;
- The IRDiRC end-to-end pathway outlines how to operationalize and sustain them across data, ethics, funding, and international alignment.
We are moving from one-off compassionate-use cases to structured guidance and emerging scalable frameworks. Full, routine scalability is not yet a reality—but the architecture for scalability is now taking shape.
Ultimately, the question is no longer “can we do this for one patient?” but “how do we do this—safely, fairly, and repeatedly—for every patient who needs it?”
How ProductLife Group Can Help
If you need support navigating the U.S. and European regulatory landscape for N-of-1 and N-of-Few personalized RNA-based therapies, you are not alone. We are here to support your initiative, meeting your challenges where they are, and pairing fit-for-purpose regulatory solutions to your needs.
This article was written by:
Register to our news and events
Go to our Events to register
Go to our News to get insights
