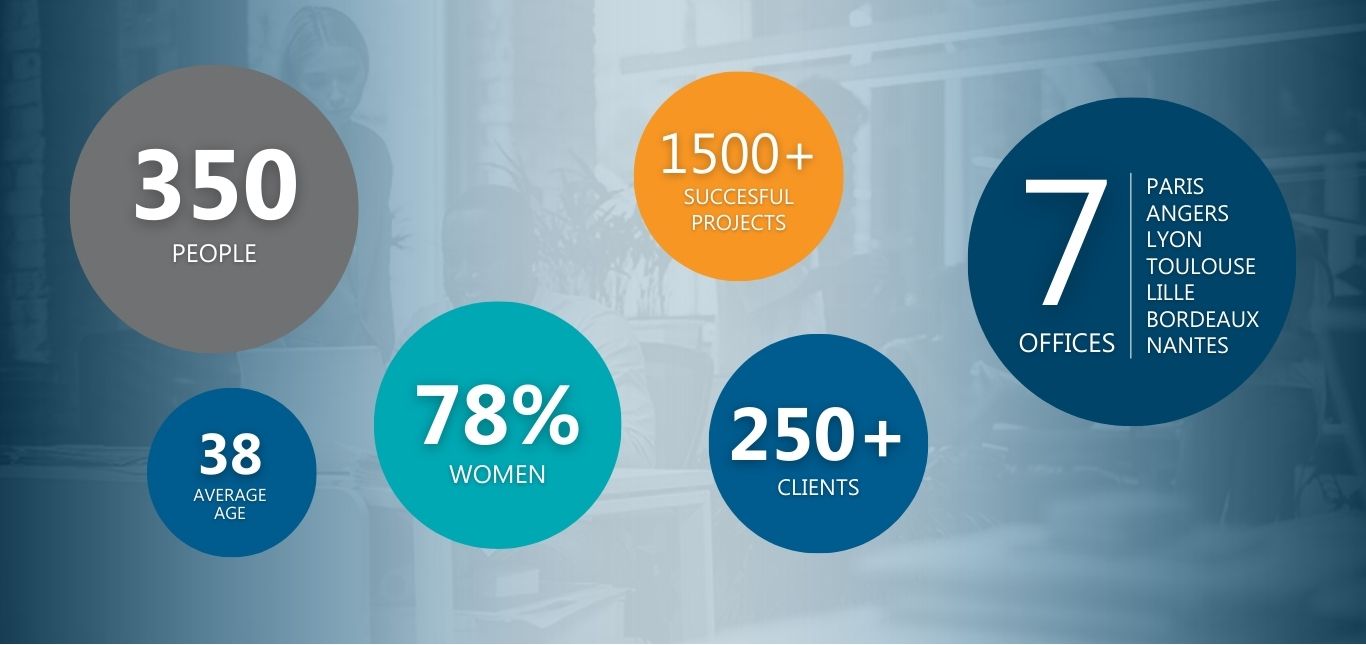
Key figures
Our Certifications


RNI
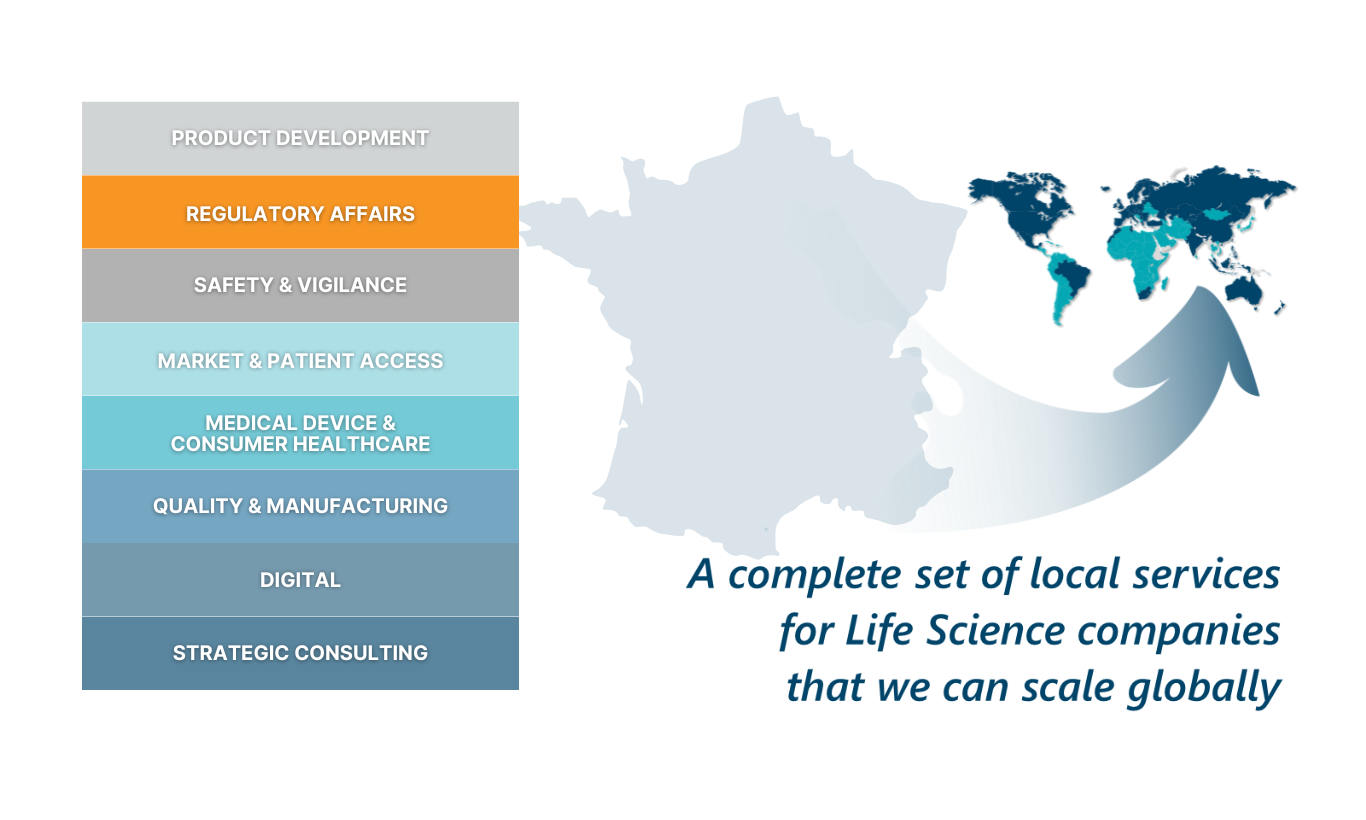
RNI is a consulting firm founded in 2006 that offers in-depth expertise in the field of regulatory and scientific affairs for nutrition, health and beauty products.RNI bridges the gap between countries so that you can achieve your domestic and international goals
Our objective is and will always be the same:
Thinking beyond mere compliance, addressing your developments and projects, even the most complex ones, in a bold and strategic way.
Advising you at every stage of your product development and helping you towards innovation in total efficiency.
Expertise
- Training : RNI is an International leader in regulatory and scientific affairs and offers a range of training courses dedicated to your professional upgrading or development needs.
- Scientific & Toxicology expertise : To provide you with support in your projects, in drafting your promotional arguments and your scientific, medical, toxicological or safety dossiers for submission to the authorities, or for marketing authorisation of your products.
- Regulatory Strategy : to advise you at each stage of development of your products and effectively support you in innovation.
- Merger and acquisition – Due diligence – M&A : Acquiring a new trademark, new factory or new business is a calculated risk. Regulatory due diligence and an acquisition audit are essential to identifying the regulatory compliance and potential of your target.
- International Expertise : RNI supports you in your international export projects.
- Quality audit : RNI will support you, stand in for you, or assist you with any internal or external quality audit
- Reg&Co : A new all-in-one regulatory and design solution. RNI’s experts understand the challenges your company faces with product development, regulatory requirements, design, marketing, and project coordination. With ReG&Co, we deliver an All-In-One solution, providing fully compliant labels for your domestic markets and abroad for your expansions.
- Advocacy : RNI engages with public decision-makers and regulatory authorities to represent and advocate for the interests of companies and the profession. Our experts monitor, anticipate, and prepare for regulatory changes through targeted training and knowledge sharing initiatives.
- Partners : RNI actively participates in leading European and International trade and scientific associations, supporting collective initiatives, advocating for key positions, and advancing ideas within the profession. This engagement enables RNI to contribute to shaping European and international regulations

Nextep
Nextep deploys its services internationally, and in particular in Europe, as a founder and member of the Medvance network.
Expertise
Market Access :
- Market access strategy for European and national markets
- Generation of clinical evidence, real-world data & new data collection methodologies
- Clinical and economic evaluation & access to reimbursement in France and Europe
- Price negotiation, new contracting modalities, and stakeholder engagement
Public Affairs :
- Advocacy & coalitions
- Lobbying and public policy evolution
- Regional territories and local actions
- Communication and brand awareness
- Studies and surveys
Strategy & Transformation :
- Patient care pathway
- Strategy and research
- Organizational transformation
- Pre-launch and product launch
- Strategy and marketing
- Healthcare professional engagement
Training and Publications :
- Dedicated “Master Classes” to address the challenges of healthcare system transformation
- Analytical briefs anticipating key issues in the pharmaceutical sector
Nextep is certified ISO 26 000:
Associations:

Key figures
1999
Foundation year
2500+
Projects delivered
1000+
Publications

Stragen Services
Expertise
Complete services or tailored solutions for pharmacovigilance and clinical development strategy
Pharmacovigiliance :
- Registration/updates in EudraVigilance: Company profile, QPPV, xEVMPD
- QPPV responsibility: Global, EU, and/or local levels
- Preparation & maintenance of the company Pharmacovigilance System Master File
- Preparation & maintenance of a validated and ICH-E2B compliant safety database
- Risk management activities including case processing, safety reports, literature review, signal detection activities
- Quality Control of Pharmacovigilance activities
- Coordination with the customer Quality, Regulatory and Legal departments for fulfilling these activities
Clinical Affairs :
- Clinical development programme definition and implementation
- Coordination and management of Contract Research Organisations (CROs) for clinical studies
- Support for definition of regulatory strategy
- Medical data review
- Medical and scientific support for regulatory submission preparation and planning
- Clinical expert reports
- Coordination of compassionate use programme

Strategiqual
Expertise
Pharmaceuticals & Medical Devices
- Development and maintenance of GxP-compliant quality systems
- Implementation of regulatory requirements
- Outsourced process management (SOPs, audits, risk analyses)
- Risk Management
- Integration into organizational structures
- Quality, pharmaceutical, and vigilance audits
- Pharmaceutical Distribution & Transport
- Audits, shortage management plans, regulatory compliance
Inspection & Crisis Management
- Strategic and operational preparation for inspections
- Coaching for Responsible Pharmacists and their teams
- Drafting of Business Continuity Plans (BCP/IMP)
- Crisis response to regulatory authority actions or decisions
Promotional Materials & Certification
- Certification of scientific promotional materials
- Strategic organization of promotional activities
- Support for HAS certification projects
- Mock audits of promotional systems and their service providers
Training
- Tailor-made training programs for healthcare professionals
- Personalized coaching for regulatory and quality roles
Certifications


SQILS FACTORY
Expertise
Our mission: To design innovative, tailor-made, and engaging training programs that align closely with regulatory, quality, and professional challenges.
DPC-accredited courses, e-learning, in-person/remote sessions, serious games, customized content, and a digitalization studio — we leverage a blended, interactive, and hands-on learning approach to maximize training impact and meet your organizational needs.
Led by industry experts, our training sessions are genuine opportunities for experience sharing, fully adapted to your products, practices, and regulatory obligations.
New in 2025: SQILS Factory® expands its offering with fresh topics including market access, clinical development, manufacturing, public health, scientific pathology-product training, and soft skills.
Sqils Factory is certified:
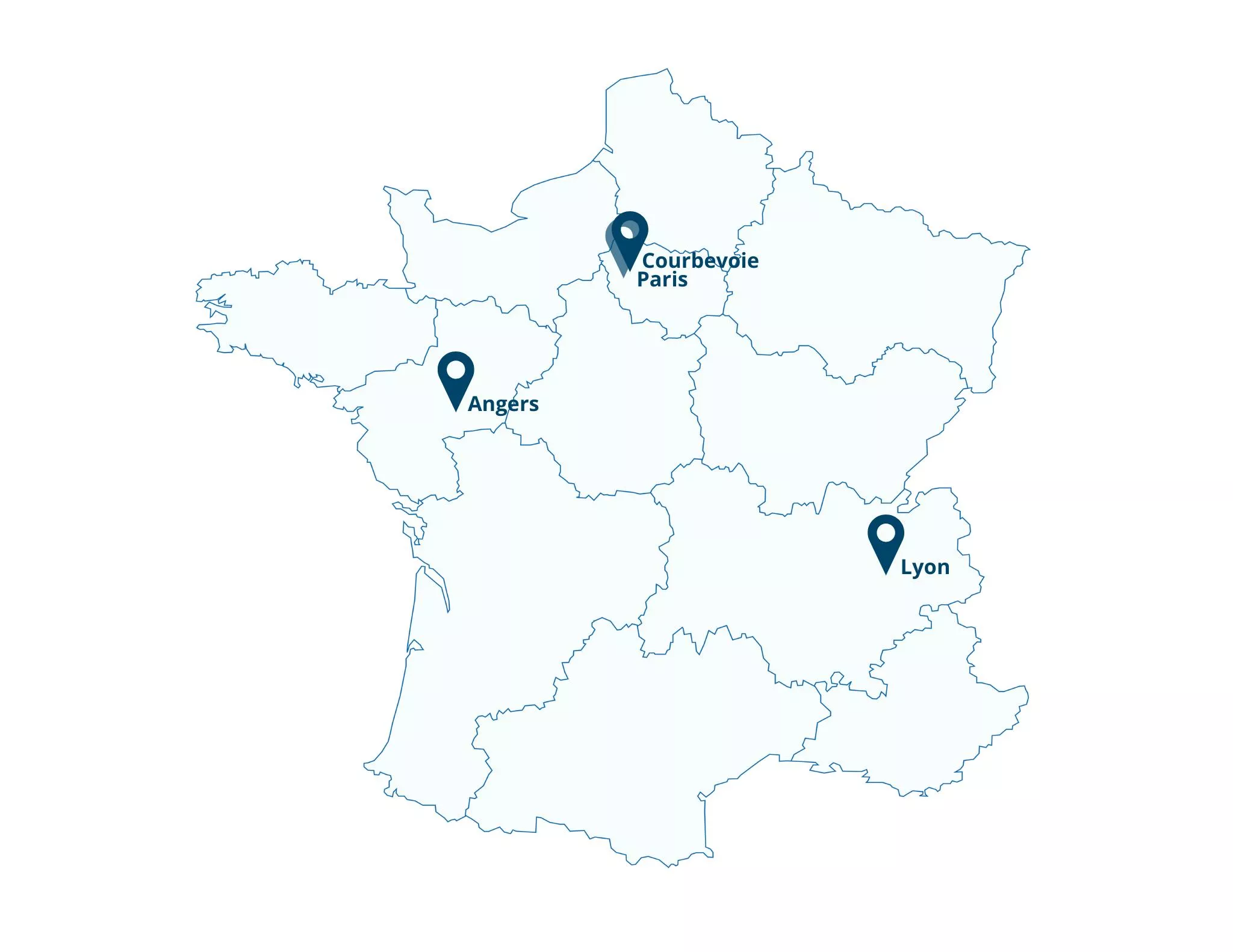
CONTACT US
Product Life France, RNI, Strategiqual/Sqils, Stragen Services
8-14 Avenue de l’Arche
92400 Courbevoie – France
Tél: +33 1 41 44 22 11
Product Life France – Office Angers
17 rue des Deux Haie
49100 Angers
Tél : +33 2 41 87 00 91
Product Life France – Office Lyon
118-126 Boulevard Marius Vivier Merle
69003 Lyon
Nextep
18-20 Place de la Madeleine
75008 Paris – France
Tél : +33 1 53 38 44 50






