DSI’s expertise: every step of the way
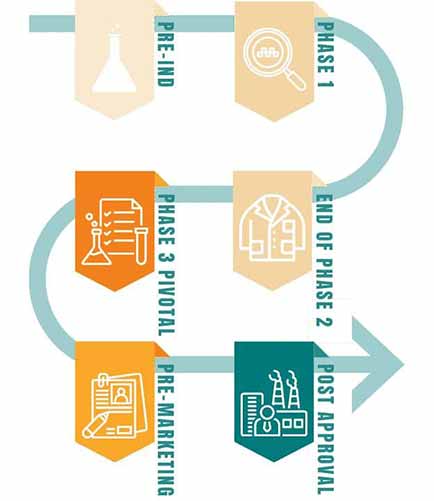
Our team of expert consultants brings decades of experience to every stage of the regulatory and product development process—from initial selection of development and manufacturing sources to design and evaluation of the investigational manufacturing process, to the scale-up for commercialization.

Practical Regulatory Affairs, CMC, and Quality Solutions
DSI provides hands-on regulatory, technical, quality, and project management consulting services for health-care companies that manufacture and/or market pharmaceutical and biopharmaceutical products.

Regulatory Affairs Services
- Regulatory Agency Representation
- Regulatory Strategy Development
- Management and Preparation of Regulatory Submissions
- Responses to Regulatory Challenges
- Expedited Drug Developments
- Next-Generation Biotherapeutics
- FDA & EU Agency Experts
- eCTD Publishing
Chemistry Manufacturing and Controls Services
- Integrated CMC Development
- Materials Characterization and Formulation Development
- Process Development, Optimization, and Validation
- Analytical Method Development, Optimization, and Validation
- Stability Program Design and Management
- Vendor/Contractor Identification and Management
- Supply Chain Assessment and Management

Quality Assurance Services
- Design, Implementation, and Remediation of Quality Systems
- Management of CMC Quality Systems
- Compliance, Vendor Qualification, and Mock Pre-approval Audits (Mock-PAI)
- Management of Compliance Situations
20+
Our experts have decades of experience in their respective fields. The average is 20 years of experience for each expert.
300+
DSI has supported 300 healthcare companies whose needs range from route synthesis to full clinical and commercial manufacturing support.
60+
with Over 60 Experts. DSI assembles multidisciplinary teams to successfully support a hundred regulatory filings in countries worldwide.
By turning to PLG’s focused and targeted CMC support, companies can realize the full potential of their projects without having to incur the costs of a larger department.
Brian Lihou
DSI General Manager